Abstract
Introduction
Chimeric antigen receptor (CAR) T cell (CART) therapy has shown to be a new and very promising therapeutical method in patients with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). The investigator-initiated Heidelberg-CAR-T-cell-trial 1 (HD-CAR-1) evaluates both efficacy and safety of escalating doses of 3 rd-generation CD19-directed CARTs comprising CD28 and 4-1BB as costimulatory molecules in patients with r/r ALL and NHL. Leukapheresis, manufacturing, administration, patient monitoring and follow-up were all conducted in-house at the Heidelberg University Hospital.
Methods
Treatment was conducted with escalating doses of autologous 3 rd-generation CARTs after lymphodepletion with fludarabine (90 mg/m 2) and cyclophosphamide (1,500 mg/m 2) in patients with r/r acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), or Non-Hodgkin's lymphoma (NHL), with subtype including diffuse large B-cell lymphoma (DLBCL), transformed follicular lymphoma (tFL) or mantle cell lymphoma (MCL). Treatment efficacy as well as occurrence of toxicities, such as cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), infections or cytopenia, were evaluated. Following prior results regarding dose-dependent efficacy and excellent toxicity profile with dose levels (DL) I, II and III (10 6, 5×10 6 and 20×10 6 CARTs/m 2), a trial amendment was approved for treatment of patients with higher CART doses (DL IV, V and VI: 5x10 7, 10x10 7 and 20x10 7 CARTs/m 2).
Results
Overall, screening was performed for 32 patients. Two patients were considered screening failures due to rapidly progressive disease (PD) and uncontrolled hepatitis B infection, respectively; leading to 30 patients enrolled in the study. The HD-CAR-1 product was given to 27 patients (12 patients with ALL, four with CLL, four with MCL, five with DLBCL, and two with FL) in different dose levels (six patients with DL I, six patients with DL II, eight patients with DL III, 5 patients with DL IV, 2 patient with DL V). The CART product was not given to two patients due to PD and lethal septic complication, respectively. Severe CAR-T toxicity was rare, as only two patients developed CRS ≥ III°. Both patients received treatment with tocilizumab, while one was additionally treated with steroids. No ICANS ≥ III° were reported in the study.
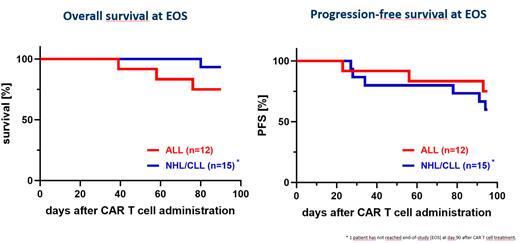
54% of patients achieved a complete response (CR) with an overall response rate (ORR) of treated patients of 65%. In a subgroup response analysis, an ORR of 92% (83% CRs) with 50% of all patients achieving MRD-negative CR was seen in ALL patients. In the NHL/CLL cohort, an ORR of 43% and a CR rate of 29% were observed. Figure 1 displays OS and PFS results till the end of study (EOS, day +90).
Conclusion
Academic CART production was feasible for all enrolled patients. Patients responded clinically to treatment and CARTs displayed a highly favorable safety profile. Overall, HD-CAR-1 accounts for clinical evaluation of 3 rd generation CARTs.
Schubert: Gilead: Consultancy. Schmitt: TolerogenixX Ltd: Current Employment; Hexal: Other: Travel grant; Jazz Pharmaceuticals: Other: Travel grant; Therakos/Mallinckrodt: Research Funding. Müller-Tidow: Pfizer: Research Funding; Janssen: Consultancy, Research Funding; Bioline: Research Funding. Dreger: Riemser: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy; BMS: Consultancy; Gilead Sciences: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Bluebird Bio: Consultancy; Roche: Consultancy, Speakers Bureau. Schmitt: MSD: Membership on an entity's Board of Directors or advisory committees; TolerogenixX: Current holder of individual stocks in a privately-held company; Kite Gilead: Other: Travel grants; Bluebird Bio: Other: Travel grants; Novartis: Other: Travel grants, Research Funding; Hexal: Other: Travel grants, Research Funding; Apogenix: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal